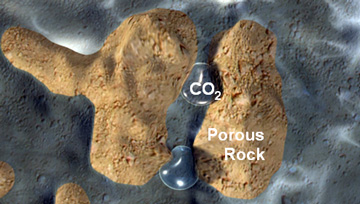
CO₂ Trapping Mechanisms
Once underground, a variety of mechanisms keep the supercritical CO₂ securely stored:
|
1 Structural/stratigraphic trapping - this is the most dominant of the trapping mechanisms. Once injected, the supercritical CO₂ can be more buoyant than other liquids that might be present in the pore space. The CO₂ will therefore percolate up through the porous rocks until it reaches the top of the formation where it meets (and is trapped by) an impermeable layer of cap-rock. With a man-made CO₂ storage site, the wells that were drilled for injection through the cap-rock would be sealed with solid physical plugs made of steel and cement, a method which is already used extensively by the natural gas storage industry. |
|
|
2 Residual trapping - this phase of trapping happens very quickly as the porous rock acts like a tight, rigid sponge. As the supercritical CO₂ is injected into the formation it displaces fluid as it moves through the porous rock. As the CO₂ continues to move, fluid again replaces it, but some of the CO₂ will be left behind as disconnected - or residual - droplets in the pore spaces which are immobile, just like water in a sponge. This is often how the oil was held for millions of years. |
|
|
3 Solubility trapping - Just as sugar dissolves in tea, CO₂ dissolves in other fluids in its gaseous and supercritical state. This phase in the trapping process involves the CO₂ dissolving into the salt water (or brine) already present in the porous rock. Just as a bottle of fizzy water is actually slightly heavier than the same bottle filled with still water, so this salt water containing CO₂ is denser than the surrounding fluids and so will sink to the bottom of the rock formation over time, trapping the CO₂ even more securely. |
|
|
4 Mineral trapping - the final phase of trapping results from the fact that when CO₂ dissolves in water it forms a weak carbonic acid (like mineral water or orange juice). Over a long time, however, this weak acid can react with the minerals in the surrounding rock to form solid carbonate minerals, much like as shellfish use calcium and carbon from seawater to form their shells or, on a far grander scale, how the White Cliffs of Dover were formed. This process can be rapid or very slow (depending on the chemistry of the rock and water in a specific storage site) but it effectively binds CO₂ to the rock. These trapping processes take place over many years at different rates from days to years to thousands of years, but in general, geologically stored CO₂ becomes more securely trapped with time.Demonstrations of various geological storage of CO₂ are already being carried out in a range of projects of varying scale. Three industrial-scale projects, which inject a minimum of around 3,000 tonnes a day of CO₂, have been under way for several years. |
|
|
The next step will be to increase the scale of storage; this will require better mapping of sources and sinks, and for storage sites to be selected, operated and monitored in a standardised way. These challenges, which can be met, will need to be carried out with the support of regulators, investors and the public.
|
|
Learn more about CO₂ capture and storage by visiting www.ccsbrowser.com
Final results book available.
View...
A summary of 20 years of CCP - as shown at GHGT-15. View...
Publication Downloads on CO₂ Capture
Background Information on CO₂ Capture
FAQs on CO₂ Capture
CCP Activities on CO₂ Capture
Publication Downloads on storage, monitoring and verification (SMV)
Background Information on storage, monitoring and verification (SMV)
FAQs on storage, monitoring and verification (SMV)
CCP Activities on storage, monitoring and verification (SMV)
Publication Downloads on policies and economics
FAQs on policies and economics
CCP Activities on policies and economics